VISUAL COMMUNICATION OF CHEMISTRY |
| |
|
|
VISUALS FOR TEACHING BASIC CONCEPTS OF SOLID STATE CHEMISTRY
Examples of visuals for teaching and learning solid state chemistry in an mixed media environment, which I produced between 2001-2011, while working with Prof. Dr. Reinhard Nesper at the ETH Zurich. |
| |
The cubic closest-packing of spheres |
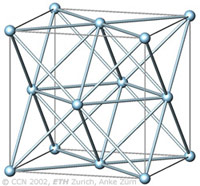 |
The cubic closest-packing of spheres (ccp) is one of the most economic ways to fill space with spheres of equal size.
The arrangement of the spheres in the cubic closest-packing corresponds to the face-centered cubic arrangement (fcc), as visualized in the figure left (light blue).
A closest-packing of n spheres has n octahedral voids (red, images below), and 2n tetrahedral voids (orange, images below). The tetrahedral voids have a primitive cubic arrangement (orange), the octahedral voids have a ccp arrangement (red). |
| |
|
|
The cubic closest-packing of spheres and the Halite crystal structure type (NaCl) |
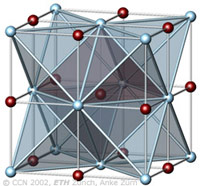 |
A closest-packing of n spheres has n octahedral voids (red), and 2n tetrahedral voids (orange). Many crystal structures can be derived from the closest-packings of spheres by occupying the octahedral and tetrahedral voids. In the NaCl-structure the Cl- ions have the arrangement of the ccp sphere packing (light blue). All octahedral voids are occupied by Na+ ions (red), which therefore also have an ccp pattern. All tetrahedral voids are empty. |
| |
|
|
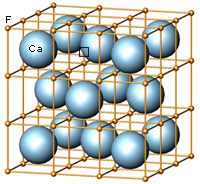
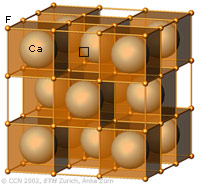
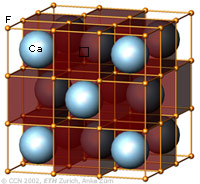
The cubic closest-packing of spheres and the Fluorite crystal structure type (CaF2) |
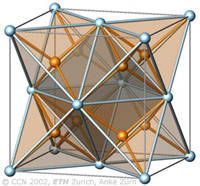 |
A closest-packing of n spheres has n octahedral voids (red), and 2n tetrahedral voids (orange). Many crystal structures can be derived from the closest-packings of spheres by occupying the octahedral and tetrahedral voids.
In the CaF2 structure type the Ca2+ ions have the arrangement of the cubic closest-packing of spheres (light blue). All tetrahedral voids are occupied by F- ions (orange), and all octahedral voids are empty. |
| |
|
|
 |
 |
 |
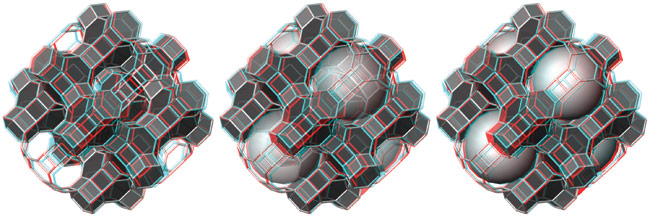
Computer generated models representing aspects of the Halite and Fluorite crystal structure types. Anke Zürn, Project CCN, ETH Zurich, 2002 |
| |
COMMUNICATING THE POSSIBLE SPATIAL ARRANGEMENTS OF ATOMS IN CRYSTAL STRUCTURES: RED-GREEN STEREO IMAGES
Paper based red-green stereo glasses are inexpensive, and small red-green stereo images are easy to download on different devices. Thus red-green stereo images are well suited for web based communications, allowing students to experience also complicated spatial arrangements, such as the possible atom positions in crystals. |
| |
 |
Computer generated models representing aspects of the Faujasite crystal structure type. Please use red-green stereo glasses. Anke Zürn, ETH Zurich, 2004 |
| |
PAST EVENTS |
einfach komplex
Bildbäume und Baumbilder in der Wissenschaft
Martin Brändle (ETH Zurich) and me, we were invited to produced visuals to dendrimers in chemistry for the exhibition. More visuals will be available here soon.
Thank you Martin! It was great fun.
30. April - 4. September 2005
Museum für Gestaltung Zürich
Ausstellungsstr. 60
CH-8005 Zürich
Kurator: Andres Janser
Öffnungszeiten:
Dienstag–Donnerstag 10–20 Uhr
Freitag–Sonntag 10–17 Uhr,
Montag geschlossen
Publication

einfach komplex - Bildbäume und Baumbilder in der Wissenschaft
Museum für Gestaltung Zürich
Barbara Bader, Andres Janser, Marius Kwint (Hg.)
2005, ISBN 978-3-907265-02-4
With texts by Barbara Bader, Andres Janser, Marius Kwint, and case studies by Jeffrey Heer, Martin Kemp, Nick Lambert, Astrit Schmidt-Burkhardt, Kelley Wilder and Richard Wingate |
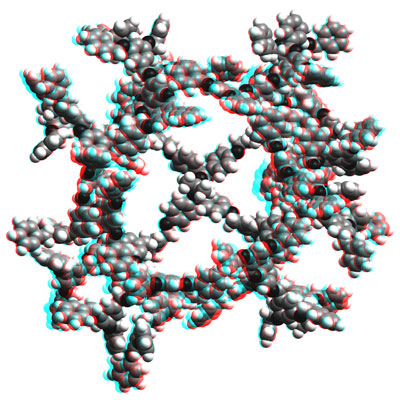
http://www.chab.ethz.ch/Rot-grün Stereobild eines Kalottenmodells der mit Molekülmechanik (CFF91-Kraftfeldansatz) optimierten Struktur des G4 TADDOL-Dendrimers [1]. Die Struktur entspricht lokalen Minima auf der Potentialfläche. Sie wurde mit dem Programm Cerius2 Version 4.6 (Accelrys Inc.) berechnet und dargestellt. Martin Brändle, Anke Zürn, ETH Zürich, © 2005
[1] (R,R)-a,a,a',a'-tetraaryl-1,3-dioxolan-4,5-dimethanol mit Poly-(Benzylether)-Verzweigungen des Fréchet-Typs.
P. Beat Rheiner, Dieter Seebach, Chem. Eur. J. 5 (1999), 3221. |
|
| |
Awards
2007 Hans Herloff Inhoffen Prize by the Ernst Schering Foundation, and the commission on chemistry teaching of the German Chemical Society (GDCh) for “pioneering work in the visualization of chemical structures”.
Publications
A. Zürn, R. Nesper
"3D stereo images for web-based teaching"
Proceedings, ICNEE 2004, Neuchâtel
A. Zürn, R. Nesper, S. P. Piotto
"Teaching Chemistry: The Challenge to Visually Communicate Complex Systems"
E-Learn 2003, Phoenix, Arizona, USA, 7. - 11. November 2003
S. P. Piotto, A. Zürn, R. Nesper
"Novel tools for combining research and teaching: inorganic and structural chemistry"
E-Learn 2003, Phoenix, Arizona, USA, 7. - 11. November 2003
S. Piotto, A. Zürn, W. Uhlig, C. Mensing, B. Ruttimann, R. Nesper
"New media for teaching and communicating inorganic chemistry: The projects CCN and CCI at the Department of Chemistry and Applied Biosciences, ETH Zurich"
Chimia 2003, 57, 94-98 |
| |
|
|